Sommaire de la page
Laboratoire de Virologie Moléculaire - UPR CNRS 9002
Team
Team leaders
| Olivier ROHR | Professor Unistra | olivier.rohr[at]unistra.fr |
| Christian SCHWARTZ | Assistant professor, MD, PhD, HDR, Unistra | schwartz.christian[at]unistra.fr |
Team members
| Clémentine Wallet | Assistant professor, PhD, Unistra | clementine.wallet[at]unistra.fr |
| Thomas Loustau | Non permanent assistant professor | thomas.loustau[at]unistra.fr |
| Léo Di Jorio | Technician | leo.dijorio[at]unistra.fr |
| Ghina El Soufi | PhD student | ghina.elsoufi[at]unistra.fr |
| Kashif Muhammad | PdH student | muhammad.kashif[at]etu.unistra.fr |
Research activities
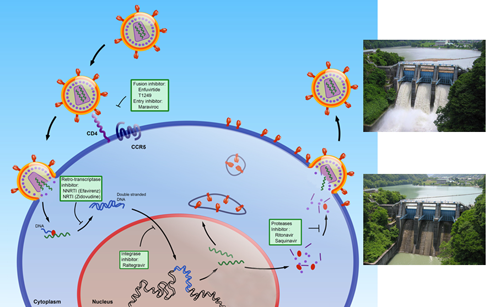
The persistence of latently HIV-infected cellular reservoirs harboring replication-competent proviruses, despite prolonged treatment with HAART (Highly Active Anti-Retroviral Therapy), represents a major hurdle to virus eradication. These latently infected cells are insensitive to HAART and escape the host immune response. They are a permanent source for virus reactivation and lead to a rebound of the viral load after interruption of HAART. Activation of HIV gene expression in these cells combined with an effective HAART has been proposed as an adjuvant therapy that could lead to the elimination of the latently infected reservoirs.
We are studying the molecular mechanisms regulating HIV-1 transcriptional latency and reactivation of HIV-1, in its natural context represented by the provirus integrated in the cellular genome and organized into chromatin. More precisely, our investigations focus on the cellular transcription factor CTIP2 and its functions on the formation and the persistence of the latently infected reservoirs.
Selection of Scientific outputs Identification and characterization of CTIP2-associated complexes as key regulators of the establishment and the persistence of HIV-1 latently infected reservoirs
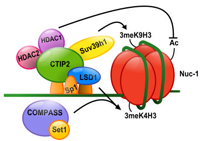
LSD1 cooperates with CTIP2 to promote HIV-1 transcriptional silencing (Contribution to the establishment of latenly infected HIV-1 reservoirs)- Le Douce et al., Nucleic Acids Res. 2012
Microglial cells are the main HIV-1 targets in the central nervous system (CNS) and constitute an important reservoir of latently infected cells. Establishment and persistence of these reservoirs rely on the chromatin structure of the integrated proviruses. We have previously demonstrated that the cellular cofactor CTIP2 forces heterochromatin formation and HIV-1 gene silencing by recruiting HDAC and HMT activities at the integrated viral promoter. In this article, we reported that the histone demethylase LSD1 represses HIV-1 transcription and viral expression in a synergistic manner with CTIP2. We showed that recruitment of LSD1 at the HIV-1 proximal promoter is associated with both H3K4me3 and H3K9me3 epigenetic marks. Finally, our data suggest that LSD1-induced H3K4 trimethylation is linked to hSET1 recruitment at the integrated provirus.
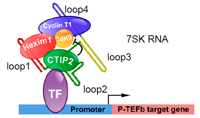
CTIP2 is a negative regulator of P-TEFb (Contribution to the persistence of the reservoirs) - Cherrier et al., PNAS 2013
The positive transcription elongation factor b (P-TEFb) is involved in physiological and pathological events including inflammation, cancer, AIDS, and cardiac hypertrophy. The balance between its active and inactive form is tightly controlled to ensure cellular integrity. Recruited by the HIV-1 Tat transactivator, P-TEFb is the cellular key factor needed to activate HIV-1 gene transcription. We have reported that the transcriptional repressor CTIP2 is a major modulator of P-TEFb activity. CTIP2 co-purifies and interacts with an inactive P-TEFb complex containing the 7SK snRNA and HEXIM1. CTIP2 associates directly with HEXIM1 and, via the loop 2 of the 7SK snRNA, with P-TEFb. In this nucleoprotein complex, CTIP2 significantly represses the Cdk9 kinase activity of P-TEFb. Accordingly, we show that CTIP2 inhibits large sets of P-TEFb- and 7SK snRNA-sensitive genes. In hearts of hypertrophic cardiomyopathic mice, CTIP2 controls P-TEFb-sensitive pathways involved in the establishment of this pathology. Taken together, our results strongly suggest that CTIP2 controls P-TEFb function in physiological and pathological conditions including HIV-1 infection.
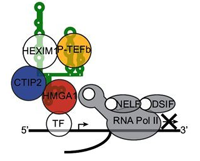
HMGA1 recruits CTIP2-repressed P-TEFb to the HIV-1 and cellular target promoters (Contribution to the persistence of the reservoirs) – Eilebrecht et al., Nucleic acids Res. 2014
As state above, active positive transcription elongation factor b (P-TEFb) is essential for cellular and human immunodeficiency virus type 1 (HIV-1) transcription elongation. CTIP2 represses P-TEFb activity in a complex containing 7SK RNA and HEXIM1. The inactive 7SK/P-TEFb small nuclear RNP (snRNP) has been detected at the HIV-1 core promoter as well as at the promoters of cellular genes, but a recruiting mechanism still remains unknown to date. In this article, we have shown global synergies between CTIP2 and the 7SK-binding chromatin master-regulator HMGA1 in terms of P-TEFb-dependent endogenous and HIV-1 gene expression regulation. In addition, chromatin immunoprecipitation experiments revealed a significant loss of CTIP2/7SK/P-TEFb snRNP recruitment to cellular gene promoters and the HIV-1 promoter on HMGA1 knock-down. Our findings not only provide insights into a recruiting mechanism for the inactive 7SK/P-TEFb snRNP, but also contribute to a better understanding of viral latency.
Proof of concept studies demonstrating the efficiency of the reactivation strategies to reduce the reservoirs (collaboration with Carine Van Lint’s Lab, ULB, Belgium)
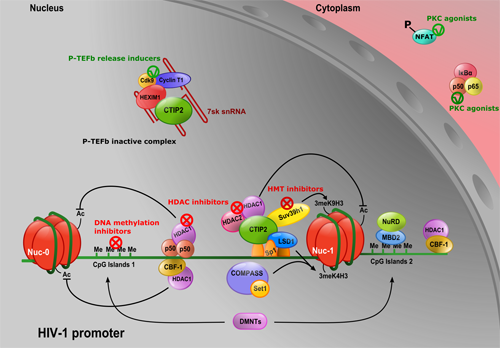
Histone methyltransferase inhibitors induce HIV-1 recovery in resting CD4+ T cells from HIV-1+ HAART-treated patients- Bouchat et al., AIDS 2012
Reactivation of HIV-1 expression in persistent reservoirs together with an efficient HAART has been proposed as an adjuvant therapy aimed at reaching a functional cure for HIV. Previously, H3K9 methylation was shown to play a major role in chromatin-mediated repression of the HIV-1 promoter. In this article, we have evaluated the therapeutic potential of histone methyltransferase inhibitors (HMTIs) in reactivating HIV-1 from latency.
We have demonstrated, for the first time, that chaetocin (SUV39H1 inhibitor) induced HIV-1 recovery in 50% of CD8-depleted PBMCs cultures and in 86% of resting CD4 T-cell cultures isolated from HIV-1-infected, HAART-treated patients, whereas BIX-01294 (G9A inhibitor) reactivated HIV-1 expression in 80% of resting CD4 T-cell cultures isolated from similar patients. Moreover, we showed that combinatory treatments including one HMTI and either the histone deacetylase inhibitor suberoylanilide hydroxamic acid or the non-tumor-promoting NF-κB inducer prostratin had a higher reactivation potential than these compounds alone. Our results constitute a proof-of-concept for the therapeutic potential of HMTIs in strategies aiming at reducing the pool of latent reservoirs in HIV-infected, HAART-treated patient
An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression – Darcis et al., PLoS Path 2015
The persistence of latently infected cells in patients under combinatory antiretroviral therapy (cART) is a major hurdle to HIV-1 eradication. Strategies to purge these reservoirs are needed and activation of viral gene expression in latently infected cells is one promising strategy. Bromodomain and Extraterminal (BET) bromodomain inhibitors (BETi) are compounds able to reactivate latent proviruses in a positive transcription elongation factor b (P-TEFb)-dependent manner. In this study, we tested the reactivation potential of protein kinase C (PKC) agonists (prostratin, bryostatin-1 and ingenol-B), which are known to activate NF-κB signaling pathway as well as P-TEFb, used alone or in combination with P-TEFb-releasing agents (HMBA and BETi (JQ1, I-BET, I-BET151)). Using in vitro HIV-1 post-integration latency model cell lines of T-lymphoid and myeloid lineages, we demonstrated that PKC agonists and P-TEFb-releasing agents alone acted as potent latency-reversing agents (LRAs) and that their combinations led to synergistic activation of HIV-1 expression at the viral mRNA and protein levels. Mechanistically, combined treatments led to higher activations of P-TEFb and NF-κB than the corresponding individual drug treatments. Importantly, we observed in ex vivo cultures of CD8+-depleted PBMCs from 35 cART-treated HIV-1+ aviremic patients that the percentage of reactivated cultures following combinatory bryostatin-1+JQ1 treatment was identical to the percentage observed with anti-CD3+anti-CD28 antibodies positive control stimulation. Remarkably, in ex vivo cultures of resting CD4+ T cells isolated from 15 HIV-1+ cART-treated aviremic patients, the combinations bryostatin-1+JQ1 and ingenol-B+JQ1 released infectious viruses to levels similar to that obtained with the positive control stimulation. The potent effects of these two combination treatments were already detected 24hours post-stimulation. These results constitute the first demonstration of LRA combinations exhibiting such a potent effect and represent a proof-of-concept for the co-administration of two different types of LRAs as a potential strategy to reduce the size of the latent HIV-1 reservoirs.
Sequential treatment with 5-aza-2'-deoxycytidine and deacetylase inhibitors reactivates HIV-1 - Bouchat et al., EMBO Mol Med 2015
Reactivation of HIV gene expression in latently infected cells together with an efficient cART has been proposed as an adjuvant therapy aimed at eliminating/decreasing the reservoir size. Results from HIV clinical trials using deacetylase inhibitors (HDACIs) question the efficiency of these latency-reversing agents (LRAs) used alone and underline the need to evaluate other LRAs in combination with HDACIs. We have evaluated the therapeutic potential of a demethylating agent (5-AzadC) in combination with clinically tolerable HDACIs in reactivating HIV-1 from latency first in vitro and next ex vivo. We showed that a sequential treatment with 5-AzadC and HDACIs was more effective than the corresponding simultaneous treatment both in vitro and ex vivo. Interestingly, only two of the sequential LRA combinatory treatments tested induced HIV-1 particle recovery in a higher manner than the drugs alone ex vivo and at concentrations lower than the human tolerable plasmatic concentrations. Taken together, our data reveal the benefit of using combinations of 5-AzadC with an HDACI and, for the first time, the importance of treatment time schedule for LRA combinations in order to reactivate HIV.
Schematic representations of the team contributions :
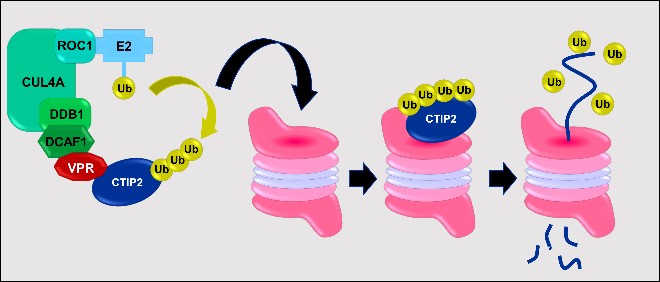
HIV-1 Vpr mediates the depletion of the cellular repressor CTIP2 to counteract viral gene silencing 2019 - Forouzanfar et al., Sci Rep. 2019
Mammals have evolved many antiviral factors impacting different steps of the viral life cycle. Associated with chromatin-modifying enzymes, the cellular cofactor CTIP2 contributes to HIV-1 gene silencing in latently infected reservoirs that constitute the major block toward an HIV cure. We report, for the first time, that the virus has developed a strategy to overcome this major transcriptional block. Productive HIV-1 infection results in a Vpr-mediated depletion of CTIP2 in microglial cells and CD4+ T cells, two of the major viral reservoirs. Associated to the Cul4A-DDB1-DCAF1 ubiquitin ligase complex, Vpr promotes CTIP2 degradation via the proteasome pathway in the nuclei of target cells and notably at the latent HIV-1 promoter. Importantly, Vpr targets CTIP2 associated with heterochromatin-promoting enzymes dedicated to HIV-1 gene silencing. Thereby, Vpr reactivates HIV-1 expression in a microglial model of HIV-1 latency. Altogether our results suggest that HIV-1 Vpr mediates the depletion of the cellular repressor CTIP2 to counteract viral gene silencing.
Inhibition of HIV-1 gene transcription by KAP1 in myeloid lineage - Ait-Ammar et al. Sci Rep. 2021
HIV-1 latency generates reservoirs that prevent viral eradication by the current therapies. To find strategies toward an HIV cure, detailed understandings of the molecular mechanisms underlying establishment and persistence of the reservoirs are needed. The cellular transcription factor KAP1 is known as a potent repressor of gene transcription. Here we report that KAP1 represses HIV-1 gene expression in myeloid cells including microglial cells, the major reservoir of the central nervous system. Mechanistically, KAP1 interacts and colocalizes with the viral transactivator Tat to promote its degradation via the proteasome pathway and repress HIV-1 gene expression. In myeloid models of latent HIV-1 infection, the depletion of KAP1 increased viral gene elongation and reactivated HIV-1 expression. Bound to the latent HIV-1 promoter, KAP1 associates and cooperates with CTIP2, a key epigenetic silencer of HIV-1 expression in microglial cells. In addition, Tat and CTIP2 compete for KAP1 binding suggesting a dynamic modulation of the KAP1 cellular partners upon HIV-1 infection. Altogether, our results suggest that KAP1 contributes to the establishment and the persistence of HIV-1 latency in myeloid cells.
COVID project Investigating the Emergence of pathogens in wastewater
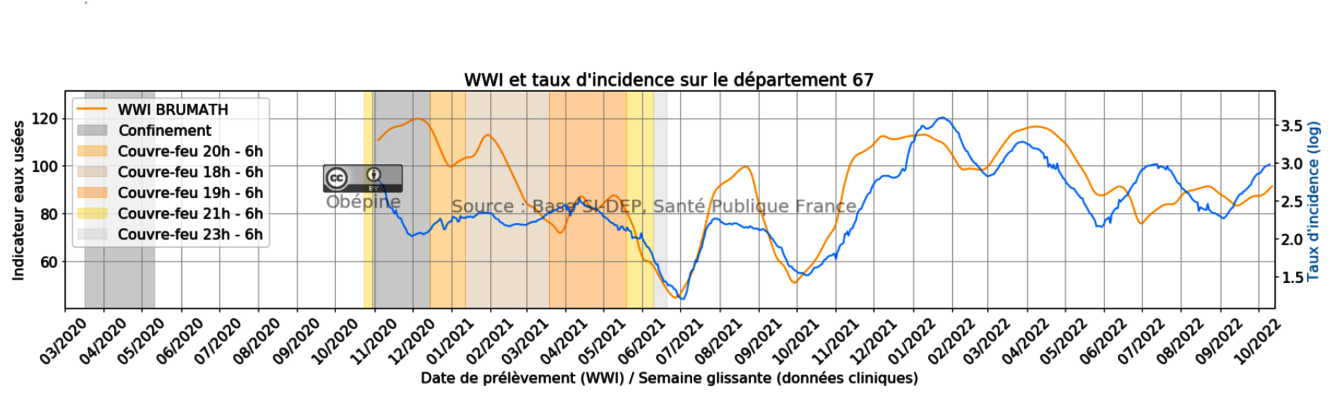
Wastewaters are currently studied as low cost and predictive indicators of pathogen emergences in populations. As part of the OBEPINE consortium, our lab analyses the wastewater from 11 wastewater treatment plants in Alsace. Our weekly-based epidemiologic survey that currently focused on SARS-CoV2 covers 400 000 persons and is representative to the region population (cities, villages, hospitals, industries …etc).
Our results are treated by a mathematic model of normalization. They allow the quantification of Sars-Cov2 circulation in population among the time. The superimposition of these data with incidence rate (blue) and hospitalization rate (red) shows the importance of our analysis for the prediction of the epidemic. In fact, we detect an increased circulation of the virus approximately two weeks before the increase of hospitalization rate. This can be explained by the fact that patient start to release virus in the stool before first symptoms appearance. Results of quantification are published each weeks on the website:
https://www.reseau-obepine.fr/donnees-ouvertes/
The current used technics for SARS-CoV2 extractions and quantifications have limits of detection around 2000-4000 genomic units per liter and do not allow efficient sequencing to follow the emergence of variants.
Strategies and scientific perspectives:
- Development of a membrane-based non-destructive method to concentrate pathogens from wastewater.
- Investigating the best storage conditions and building a national wastewater-biobank for retrospective studies on pathogens emergence.
Our collaborations :
Strategies and scientific perspectives
Our scientific strategies and perspectives are part of the work packages 2 and 3 of our H2020 RISE project.
As part of the international consortium, we are leading the work package 2. Our goal is to identify, at a global level, CTIP2-associated complexes and to define their functions in HIV-1 gene latency. These contributions will highlight new therapeutic targets that will be tested in cure strategies by our collaborators.
By Quantitative Mass Spectrometry experiments, we will identify CTIP2-associated factors. These interactions will be confirmed by Immuno-precipitation experiments and the functions by loss/gain function studies and mechanistic studies. The studies will be performed in models of latent HIV-1 and compared to primary resting CD4T cells that are the main reservoirs of latent HIV-1 infection.
In a second phase, the newly identify regulators of HIV-1 latency will be targeted by specific drugs to evaluate the efficiency of reactivation in cellular models.
Finally, the efficient treatments will be tested on HIV-1 reservoirs from patients with well characterized virological- and immunological profiles.